Amino Acids: Structure & Classification
Mastering the building blocks of life (and passing the exam).
1. General Structure
Every Amino Acid (AA) has the same skeleton (except Proline). If you can't draw this, drop out.
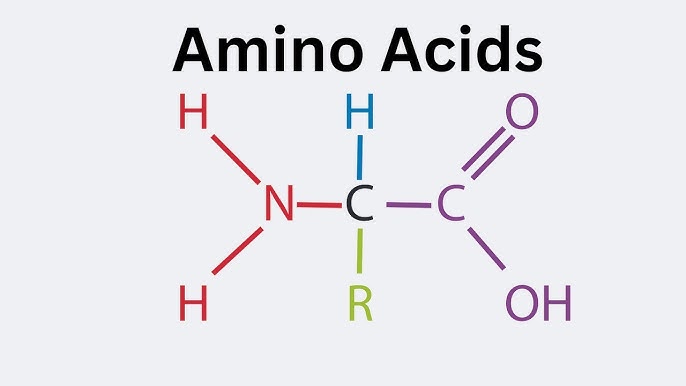
- α-Carbon: The center.
- Carboxyl Group (COOH): Acidic.
- Amino Group (NH2): Basic.
- Side Chain (R): The variable that makes them unique.
- H atom.
High Yield Alert
- Number of AA: 20 standard AA enter protein structure.
- The 21st AA: Selenocysteine. (Examiners love asking this. It’s encoded by a stop codon).
- Proline: The only Imino Acid (NH group instead of NH2). It breaks the α-helix structure.
🧪 Part 2: Classification (The Meat)
The examiners will ask: "Enumerate Essential Amino Acids" or "Classify AA chemically." Memorize these tables.
A. Nutritional Classification
Based on: Can your body make it?
| Type | Definition | The List (Memorize This) |
|---|---|---|
| Essential | Body cannot synthesize. Must eat it. |
TV TILL PM Threonine, Valine, Tryptophan, Isoleucine, Lysine, Leucine, Phenylalanine, Methionine. |
| Semi-Essential | Synthesized, but not enough for growing children. | Arginine (the only one). |
| Non-Essential | Body synthesizes it easily. | The rest (e.g., Glycine, Alanine, Serine). |
B. Chemical Classification
Based on: What does the R-group look like?
1. Aliphatic (Straight Chain)
- Simple: Glycine (Smallest), Alanine.
- Branched (BCAA): Valine, Leucine, Isoleucine.
*Metabolized in muscle, not liver. - Hydroxy (OH): Serine, Threonine. *Site of Phosphorylation.
- Sulfur Containing: Cysteine (SH), Methionine.
- With COOH: Aspartic Acid, Glutamic Acid.
- With NH2: Lysine, Arginine, Hydroxylysine, Histidine
2. Aromatic (Rings)
- Phenylalanine: Benzene ring.
- Tyrosine: Phenol ring.
- Tryptophan: Indole ring.
3. Heterocyclic (Ring with N)
- Histidine: Imidazole ring. (Crucial for buffering).
- Proline: Pyrrolidine ring.
- Tryptophan: Indole ring.
C. Polarity Classification
Based on: Does it love water or hate it?
- Non-Polar (Hydrophobic): "The greasy ones." Mostly found in the interior of proteins. (Leucine, Valine, Proline, Phenylalanine).
- Polar Uncharged: Soluble. (Serine, Threonine, Cysteine, Glutamine).
- Polar Charged (Ionic):
- Acidic (-ve charge): Aspartic Acid, Glutamic Acid.
- Basic (+ve charge): Lysine, Arginine, Histidine.
⚡ Part 3: Properties of Amino Acids
Written Exam Material. They ask "Explain the Amphoteric property."
1. Amphoteric Property
Amino acids act as ACIDS (proton donors) and BASES (proton acceptors) depending on the pH.
- Low pH (Acidic): AA acts as a Base → accepts H+ → becomes Cation (+ve).
- High pH (Alkaline): AA acts as an Acid → donates H+ → becomes Anion (-ve).
2. Zwitterion (Dipolar Ion)
The state at a specific pH where the AA carries both positive and negative charges, but the Net Charge is ZERO.
- It does not migrate in an electric field.
- Most AA exist as Zwitterions at physiological pH (7.4).
3. Isoelectric Point (IEP)
The specific pH value at which the AA exists as a Zwitterion (Net charge = 0).
4. Peptide Bond Formation
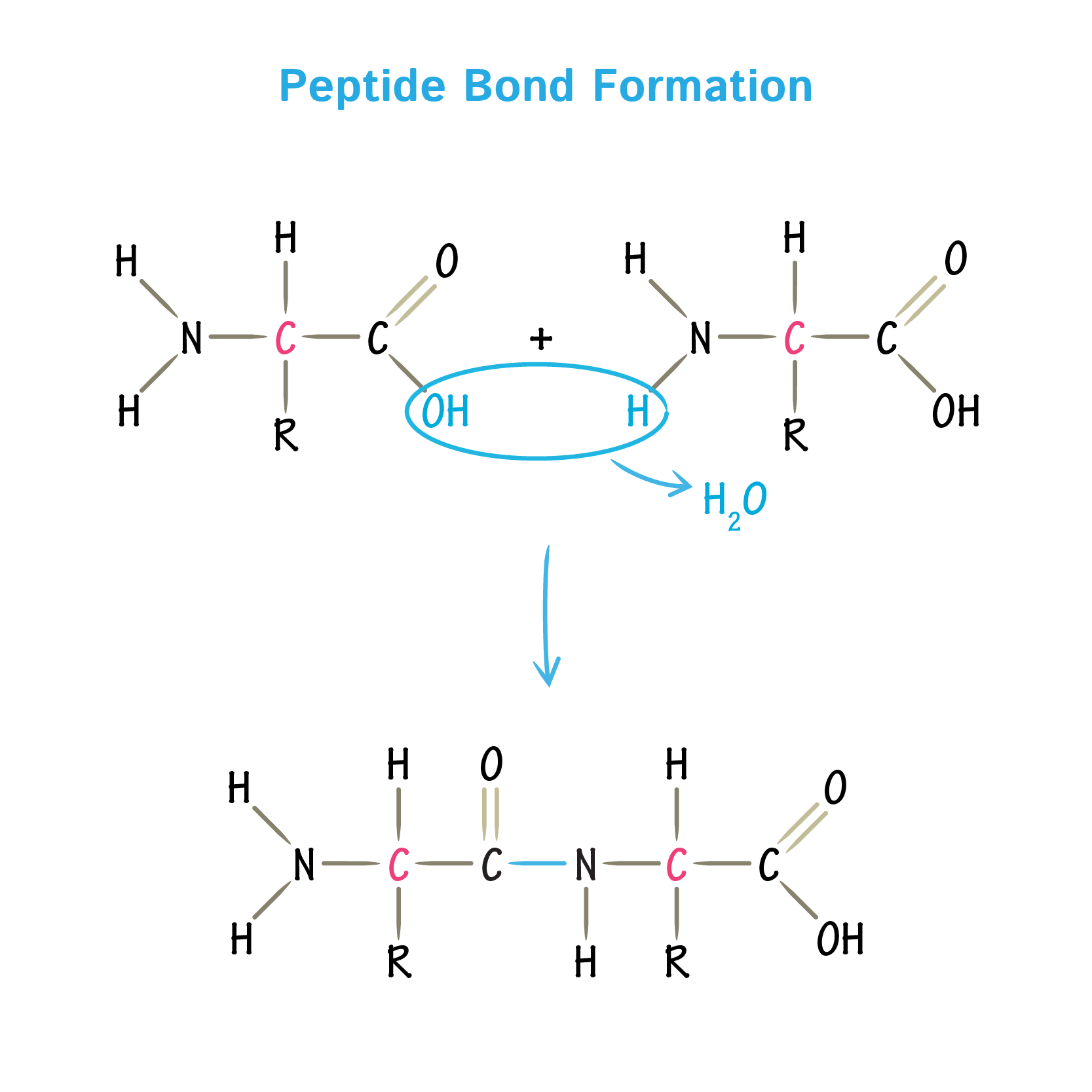
- Type: Covalent Amide bond.
- Reaction: Condensation (Removal of H2O).
- Between: α-COOH of one AA and α-NH2 of the next.
- Characteristics: Rigid, Planar, and partial double-bond character (no rotation).
🚨 Part 4: The "Exam Traps"
Directly from the Department Book & Dr. Hesham’s MCQs.
- Hydroxyproline & Hydroxylysine: Found in Collagen. Requires Vitamin C.
- Gamma-Carboxyglutamate: Found in Prothrombin (Clotting). Requires Vitamin K.
- Indole Ring → Tryptophan
- Imidazole Ring → Histidine
- Guanido Group → Arginine
- Phenol Ring → Tyrosine
🎓 Adel's Reality Check
"Don't memorize the chemical formula for Tryptophan unless you hate yourself. Just recognize the double ring (Indole). For the written exam, focus on the Classifications (Part 2) and the Amphoteric Definition (Part 3). Those are the guaranteed marks."